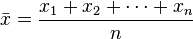I've written several posts recently about thresholding images. I was thus pretty excited to see Steve Eddins announce a new series of posts on his blog.
http://blogs.mathworks.com/steve/2016/04/28/image-binarization-new-functional-designs/
My impression from a very quick scan of the documentation is that MATLAB 2016a includes a new function imbinarize which can be called with a locally adaptive threshold option.
I don't think this will lead to a breakthrough in image processing - adaptive thresholding is not a new technique - but it will simplify coding. The new function will allow users to replace multiple lines of 'old' MATLAB code with a single command.
And in my opinion, that's why MATLAB is so useful for scientific computing. It's not that the software gives you completely new options; you can code anything in C if you want to. It's just that MATLAB makes it easy to try things without investing huge amounts of time. That's why it accelerates science.
http://blogs.mathworks.com/steve/2016/04/28/image-binarization-new-functional-designs/
My impression from a very quick scan of the documentation is that MATLAB 2016a includes a new function imbinarize which can be called with a locally adaptive threshold option.
I don't think this will lead to a breakthrough in image processing - adaptive thresholding is not a new technique - but it will simplify coding. The new function will allow users to replace multiple lines of 'old' MATLAB code with a single command.
And in my opinion, that's why MATLAB is so useful for scientific computing. It's not that the software gives you completely new options; you can code anything in C if you want to. It's just that MATLAB makes it easy to try things without investing huge amounts of time. That's why it accelerates science.